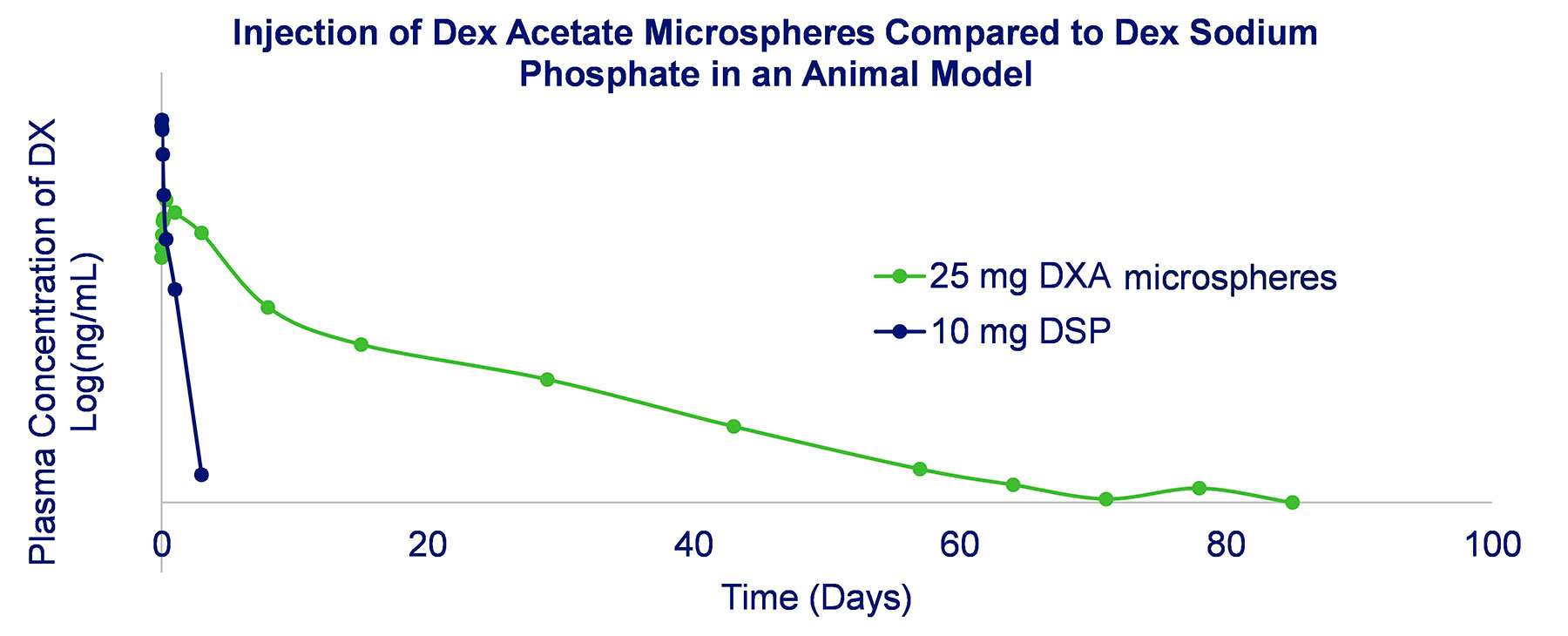
Sustained release formulations can also be prepared using injectable microspheres typically made from a resorbable polymer such as PLGA. Key capabilities include:
- Microspheres produced using proprietary or non-proprietary methods.
- Tightly controlled sizes from 2-50 µm, spherical, and non-aggregating
- Drug release profile can be engineered from ~3 to >180 days
- Compatible with a range of small molecule API’s (peptides also possible)
- Delivery at high concentration (>800mg/ml), small gauge needles (as small as 30G)
- Powder for suspension, packaged in glass vials, stored refrigerated, option for terminal sterilization