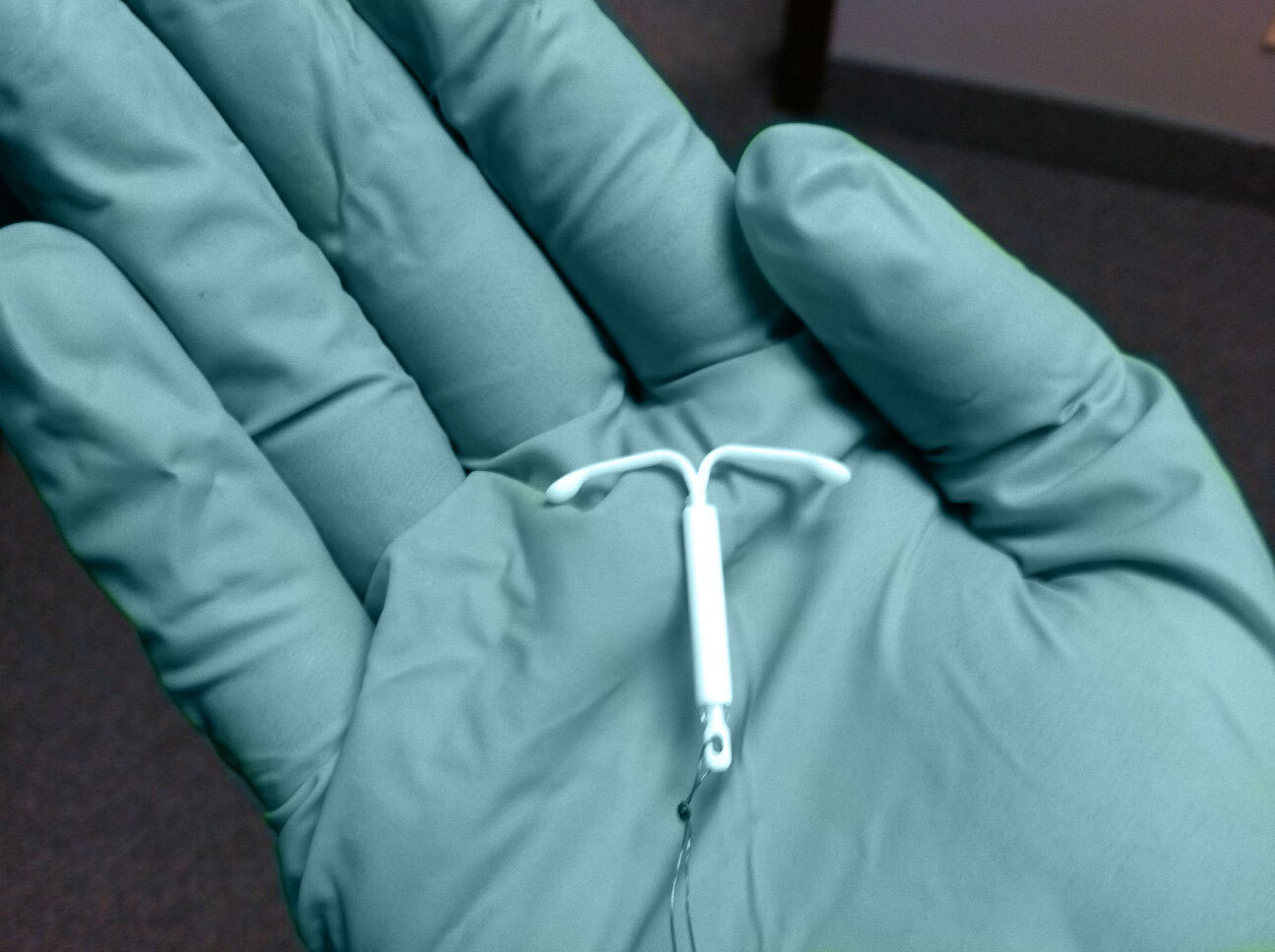
Novel drug delivery forms such as intrauterine devices (IUDs) and intravaginal rings (IVRs) and are finding increasing applications in the area of women’s health. In 2000 the FDA approved a levonorgestrel eluting IUD (Mirena®) providing contraception for up to 5 years of use. Later, use of the device was expanded to include an indication for severe menstrual bleeding more recently a smaller device (Skyla®) was approved for women who have not had children. IVRs are commercially available for contraception (Nuvaring®), hormone replacement therapy (Estring®), and to improve the rate of in vitro fertilization (Milprosa®). ProMed has also contributed to the development of novel multipurpose subcutaneous implants for contraception and HIV prevention as well as a fully bioresorbable 18-24 month contraceptive implant.